Diagnosis at your doorstep
-
- from Shaastra :: vol 01 edition 02 :: Jul - Aug 2021

A number of Indian start-ups are harnessing cutting-edge technology to take screening and diagnosis closer to patients and the point of care.
Some 15 million diabetics in India develop diabetic foot ulcers (DFUs) in their lifetime. Half of these become infected and need hospital care. About a million of them need amputation. These were probably just statistics, albeit grim ones, to Geethanjali Radhakrishnan until she visited a tertiary care hospital in Pune. A bioengineer with software experience and entrepreneurial ambitions, Geethanjali had an idea for a medical technology start-up and wanted to maximise its impact. In other words, she had the makings of a solution, and was looking for a big-enough problem to solve with it.
At the hospital, there had been patients from nearby villages. They had turned up years after an initially minor foot infection had festered and turned potentially lethal, leaving the doctors with no choice but to amputate. There were others whose fungal infection had been wrongly treated with anti-bacterials by a local doctor.

This was in spite of the fact that there has been a gold standard - the culture test - in existence for years, to suss out the nature of an infection and guide doctors towards the right treatment. But this test was the domain of well-equipped labs. "In low-resource settings, there was no lab to figure out the nature of the infection," Geethanjali says. Besides, even a lab result could take days. The patient would be lost to follow-up. Or the cost would discourage patients from testing, and doctors from prescribing the test. Treatment based on visual inspection could lead to irrational antibiotic use while crucial time was lost. For DFUs, the earlier the intervention, the greater are the chances of healing.
Geethanjali had found the problem: the absence of rapid, accurate screening for wound infections at the point of treatment. Early last year, the company she founded, Adiuvo Diagnostics, launched Illuminate, a portable, handheld, machine learning-enabled imaging device, initially targeted at DFUs. Simply put, it uses imaging and fluorescence to highlight which parts of the wound are infected, and claims to identify the broad category of infection - bacterial or fungal, gram-positive or gram-negative - with over 85% accuracy, helping doctors choose appropriate treatment and monitor healing. The turnaround time is just a couple of minutes.
MOBILE MEDTECH
Illuminate is part of an emerging area of medical technology known as point-of-care (PoC) testing. Literally, PoC techniques take testing/screening to the doctor's cabin or the patient's bedside - the places where care is typically provided. However, in countries such as India, with limited lab infrastructure, PoC may be read to include even near-care testing that is distributed beyond the large central labs - to smaller, neighbourhood labs, primary healthcare centres, or even mobile health vans.
As self-care and a focus on wellness gain currency in urban India, the term may be expanded to cover home testing, too. What's common to all scenarios is this: the solution has to be easy to transport, store and use with minimal or no additional training. The results should be quick and reliable. A single platform can be used for a range of applications. For instance, Illuminate can also be used in burn-related infections.
In the last few years, start-ups working on different types of technologies - from a lab-on-chip to breath-based screening - have emerged. "In a country as big as India, you cannot have only a centralised lab system where you - or the sample - are travelling hundreds of kilometres," says Sahil Jagnani, founder and CEO of Primary Healthtech, an IIT Guwahati-incubated start-up. "PoC is the solution." Jagnani's company has developed a light-weight and portable device that uses paper-based strip tests to measure functions of the kidney, liver and pancreas in under two minutes.
"I see a bright future in the early diagnosis market," says Jilma Peruvangat, co-founder and CEO of Pune-based Kozhnosys, which has a prototype of CanScan, a compact device that uses exhaled breath to screen for pre-symptomatic breast cancer. CanScan can potentially be placed in smaller diagnostic centres and hospitals, she says. Like Geethanjali, Jilma found the "pain point" after meeting women diagnosed in Stage 2 - that is, only after symptoms appeared. In small studies, CanScan had a 90% accuracy rate, with results in under 10 minutes, says Jilma. This needs to be borne out further in clinical validation.
Unlike in a central lab, where there may be trained staff, it may be a paramedic or a patient who is using the point-of-care diagnostics device.
UNMET NEED
Several factors make a case for such solutions. One is the growing body of evidence that for many diseases, early detection-plus-treatment results in much better clinical outcomes. The second factor is the public health advantage. The third is the battery of tests that now count as routine with the rising global burden of non-communicable diseases such as diabetes and cardiovascular disease, and the accent on wellness and self-care.
However, conventional technologies were not designed for rapid turnaround or distributed deployment. Consider PCR (Polymerase chain reaction), a molecular testing technology commercialised in the 1990s, which heralded a change in the detection of infectious diseases owing to superior sensitivity and specificity. It punched below its weight in India. Performed by a bulky machine costing lakhs of rupees, with each test costing ₹4,000-7,000, it was limited to well-capitalised central laboratories. It was an expensive diagnostic technology to acquire and run, needing trained staff. Samples had to be ferried from collection centres all over the country, without being contaminated, to central labs to maximise throughput. A result could take three to 10 days depending on the location.

Rather than rely on it, "doctors would treat empirically," says Sriram Natarajan, founder director and CEO of Molbio Diagnostics. "As a result, in spite of being the most reliable diagnostic, people could not really benefit from it." For instance, for a high-burden disease such as tuberculosis (TB), India relied on the more accessible and cost-effective - but relatively less accurate - sputum smear microscopy. In 2018, Molbio launched Truenat, India's first indigenous and fully automated PoC molecular testing platform or micro-PCR. Truenat is now used for near-care TB and COVID-19 testing, among other infectious diseases.
Or consider various cancers where early detection can lead to reduced mortality and less radical treatment. Breast mammography is not part of routine screening packages for women under 45, observes Jilma. An additional barrier is the nature of the test itself, which is seen to be painful and uncomfortable. Among the patients that she met during her research, none came in after routine screening.
For certain kinds of tests, imported PoC tech is available, but could be expensive. For instance, a good quality home cholesterol analyser costs ₹10,000-15,000 with recurring consumables (such as the strip and lancet) costing ₹1,000 per test. "This is not viable for the end-user," says Anurag Meena, co-founder and System Architect at Mumbai's Dynasense Technologies. Dynasense has developed the prototype for a home kit that could cut the per-test cost to a third.
In parallel, there is also an emerging class of "people willing to pay for convenience" in certain situations, says Dhananjaya Dendukuri, co-founder and CEO of Bengaluru-based Achira Labs. Its flagship lab-on-chip platform runs rapid immunoassays on a benchtop device. Achira wants to install this in distributed settings such as doctors' offices or in-vitro fertilisation clinics to run hormone tests that are currently in clinical validation.
THE ENABLERS
While the core diagnostic technology may have changed little in the last few decades, incremental advances in fields such as materials, electronics and computing have reportedly helped the cause of PoC. Machine learning and AI are also put to work.
Take Illuminate from Adiuvo. This is a multi-spectral imaging device. It shines light from multiple wavelengths onto the wound and collects images. Bacteria or fungi present on an infected wound fluoresce under this light, revealing infected portions. An AI algorithm matches the fluorescence to a database - generated from hundreds of trial samples - to identify and classify the infection.
Advancements in mobile diagnostics and smartphone-based imaging have helped in miniaturising multi-spectral imaging, says Geethanjali of Adiuvo. AI was used "to develop a self-training model that can quickly analyse various parameters, to help in real-time diagnostics," she says. The next step is to narrow down an infection to a specific genus such as staphylococcus, she adds.
Others, such as breath-based testing, have been helped by advances in analytical instruments. Disease can lead to a change in the concentration of organic compounds or form new compounds in exhaled breath. CanScan uses gas chromatography sensors, which are now refined enough to diagnose parts per trillion, to detect these, Jilma explains. They can potentially diagnose a range of cancers.
At Achira, building on advances in microfluidics, its lab-on-chip platform ACIX200 uses substantially lower volumes of sample and reagent to get lab-standard results and test for multiple analytes at the same time. Microfluidics are also used in Truenat's consumables.
And at Dynasense, Meena's team would like to strip down its Bluetooth-enabled device into a small block that can be attached to a regular smartphone. This way, says Meena, power and computation can be outsourced to the phone, doing away with the need for a separate screen or battery. A smartphone app will register and record readings. There is also a plan to use software from a sister company, Neodocs, to analyse the results, explain the findings, and make diet and lifestyle recommendations.
Government funding from the Department of Science and Technology, the Department of Biotechnology and the Indian Council of Medical Research is available to take start-up ideas in PoC upto proof-of-concept stage, says Rohit Srivastava, Professor of Biosciences and Bioengineering at IIT Bombay, whose lab developed the assays in Dynasense's kit. "Translational projects in PoC diagnostics are easily funded."
There is growing academic interest in PoC techniques, says Vivek Borse, Professor in IIT Guwahati's Centre for Nanotechnology. Borse set up the NanoBioSens Lab at the Centre to research PoC techniques, among other things, and is working on such a technique for oral cancer using blood and/or sputum. "India is in a sweet spot," he says. "We have plenty of patients, many researchers on PoC techniques that are in a good position to collaborate with start-ups." Borse is in talks with Jagnani of Primary Healthtech about solutions emerging out of NanoBioSens.
Manufacturing has also received a boost with the establishment in 2016 of the Andhra Pradesh Medtech Zone (AMTZ), which has manufacturing and testing facilities for medtech companies. Soft loans are offered from the Technology Development Board in the Department of Science and Technology. AMTZ has proved valuable during the pandemic as a location to ramp up manufacture of reagents and equipment for COVID-19 testing, including for Molbio's Truenat.
THE HURDLES
Even today, 80% or more of the medical technology in use is imported. And while capacity in specific areas such as RT-PCR test kits or hospital equipment such as ventilators may have been ramped up in response to the pandemic, a fully developed ecosystem in medtech manufacturing is still something of a work in progress. Sriram of Molbio says that it continues to import nucleotides, which are the primers or starting point for all Truenat tests. "It needs expertise and infrastructure to make primers at consistently good quality," he says. "And while it can be done, nobody talked about doing it because the market was too small."
Geethanjali of Adiuvo points out that Illuminate needs customised LEDs of specific wavelengths, but local companies cannot support those specifications. "We depend on the U.S., China, Korea," she says. Her company also imports hardware boards, although camera chips are sourced from local vendors. For start-ups that are still in the clinical validation phase, the lack of scale can become a stumbling block to procure inputs, says Jagnani of Primary Healthtech. After scouting in vain for a supplier of a specialised electrode, his team made it themselves even though it was a time-consuming process.

Additionally, go-to-market funding is a challenge. In addition to the government, start-ups have been mostly supported, with some exceptions, by impact funding agencies such as social enterprise incubator Villgro, and Grand Challenges Canada. Jilma of Kozhnosys, who has been attempting to raise funds from angel investors for clinical validation of the CanScan prototype, says, "The usual response is that they would like to invest in companies that already have traction or a product in the basket."
Then there is the regulatory landscape that must be navigated. India has a strange dichotomy when it comes to medical technology. On the one hand, dubious tests, including imports, abound in the private market with hardly any clinical validation. At the Apollo Hospitals group, which is a big user of PoC in its health screening initiatives, Dr Sushma Pathak's team evaluates five such solutions each day, but ultimately rejects a majority of them. "While there is a need for PoC solutions, these need clinical back-up," says Pathak, Senior Program Manager, Apollo TeleHealth.
Point-of-care techniques literally take testing to the doctor's cabin or the patient's bedside.
On the other, for those who want to do it right, the regulatory process sometimes feels like a black box. "There is a need for a clear-cut process to get certification," says Jagnani of Primary Healthtech. While India's relatively recent medtech rules clearly group devices according to risk classes, the actual process follows different pathways depending on the category of device and its proposed use. For many devices, regulation is still being phased in, and companies are urged to follow voluntary certification for market acceptance and to prepare for eventual regulatory approval.
Companies too have to realise the importance of rigorous clinical validation, says Chandrasekhar Nair, Chief Technology Officer, Molbio, whose company Bigtec Labs (now a Molbio subsidiary) first envisaged the micro-PCR platform in 2005. "The PoC community must realise that it (generating evidence) is going to be a massive task especially if the goal is public health programmes," he says. It took three years for Truenat to be validated for use in India's TB programme.
There is an additional consideration with PoC tests, says Sriram of Molbio. "Unlike in a central lab, where there is trained staff, here it could be a paramedic or a patient using the test, and so there is significantly more responsibility (on the provider)." It is also necessary that they address a clear, unmet need if they truly want to make an impact, he says. Geethanjali of Adiuvo is aware of this. Her company, which has supplied Illuminate to various hospitals, is proposing a study of outcomes - such as differences in antibiotic use and time taken for wounds to close - with the use of Illuminate compared with visual inspection and culture tests in two public hospitals.
As Dhananjaya of Achira stresses, PoC approaches must be used for a limited set of tests and are not meant to entirely replace centralised testing. For instance, Truenat does not expect to replace conventional RT-PCR testing, but looks to create more accessible PCR capacity. As Molbio's Nair puts it, "no single strategy is going to fulfil the diverse needs of the country."
Handy equipment
Key technologies that support point-of-care testing.
Microfluidics/Nanofluidics

What it is used for: Manipulating fluids in channels with dimensions of tens of micrometres/nanometres.
Application: Medical diagnostics; drug discovery and delivery; artificial organs.
Used in: Lab-on-chip multiplex testing; rapid molecular tests.
Global companies in microfluidics-based testing: Abbott, Cepheid, Quidel.
Biosensors/Nanobiosensors

What it is: Biosensors are devices designed to detect or quantify biochemical molecules such as a DNA sequence, protein or metabolite. Nanobiosensors are biosensors in nano dimensions to detect miniscule quantities.
Application: Food and water contamination; medical diagnostics.
Used in: Routine blood tests; infectious disease detection; breath analysis.
Global companies using them for diagnostics: Nemaura Medical, HemoCue, Masimo, RoboScientific.
Mobile diagnostics
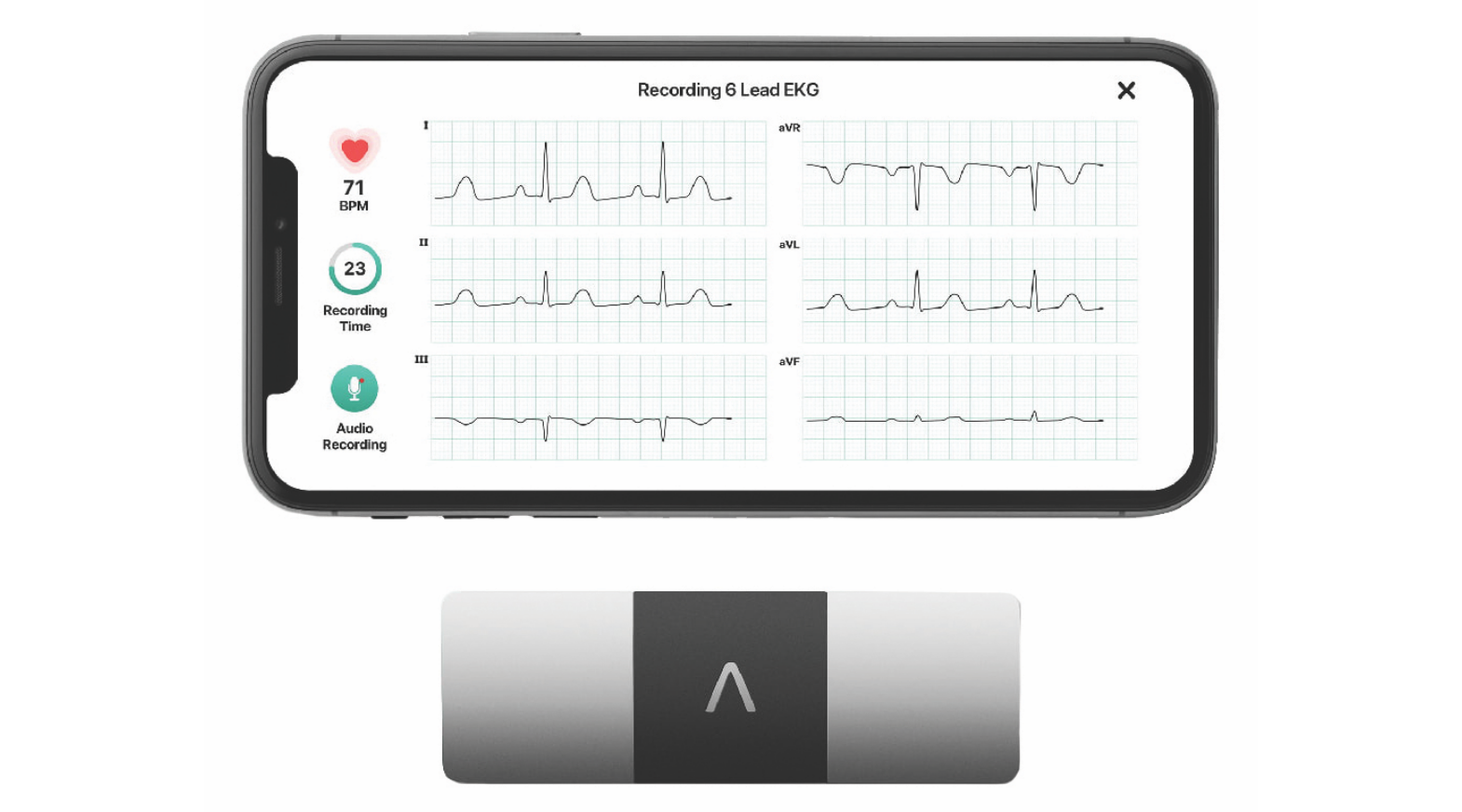
What it is: Adapting smartphones to sensing and diagnostics needs related to healthcare to leverage their portability, connectivity and functionality for near-care or PoC testing.
Application: Wearables for monitoring oxygen and heart rate; whole-body imaging; wound assessment.
Global companies in this space: AliveCor, Butterfly iQ, HealthIO, Luminostics.
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH