Untangling Alzheimer’s disease
-
- from Shaastra :: vol 03 issue 09 :: Oct 2024
Despite setbacks, the diagnosis and treatment of Alzheimer’s disease move ahead due to advances in blood-based markers.
As the world's population ages, healthy ageing will become a major concern of public policy and medical attention. Of particular interest in an ageing population is mental health. All of us know people — close relatives and friends — who display signs of dementia. In our conversations and interactions, we are puzzled and either label them incorrectly or bin them all into one category as cognitive decline. This is analogous to what we used to do some decades ago with regard to cancers; we just called them cancers of a particular tissue or organ. Today, unlike then, not only have we dispensed with this broad characterisation, we have a molecular profiling of different cancers in great detail. This has led to a substantial understanding, for example, of each sub-type of leukaemia and its origin. This, in turn, has led to specific treatments of each type of leukaemia with drugs, antibodies or more novel therapies involving gene-editing. Similarly, today we see the study of mental health moving from coarse characterisation to finer definitions that relate genetic and molecular characterisation with patient presentation and outcomes. This is leading to a closer connection of molecular characterisation to management and treatment. It will be fair to bet that as mental health problems increase all over the world over the next few decades, management and treatment will also get better, combining molecular tools that allow both early detection and better, personalised treatment.
But, first, it is important to appreciate the scale of the potential problem. Today, 88% of India's population is under the age of 50, reflecting a relatively young demographic profile compared to many other countries. Given our large population, the 12% of our population above 50 years of age is estimated at 168 million (16.8 crore) people. This is more than the populations of Germany and France put together. This is the 'older' population that will be susceptible to mental health illnesses and dementias at a significant level of risk. The World Health Organization (WHO) estimates that around 5-8% of people over the age of 60 are affected by dementia at any given time, though the risk increases as people age.
Assuming India's ageing population is subject to similar dementia risk factors, close to 12 million people (1.2 crore) of India's current population could be at risk of developing dementia as they continue to age, although this estimate may vary based on regional, genetic and health factors specific to India.
RISKS AND PROTECTION
While dementia risk increases significantly with age, in India risk factors are influenced by several factors, both unique to us and shared globally. Understanding these influences provides insights into how ageing populations, particularly those over age 50, are affected. India's ageing population is growing, with improving life expectancy (currently around 70 years). But the longer that people live, the higher their risk of cognitive decline. Our ageing population, those above 60, is projected to nearly double in the coming decades, further increasing the burden of dementia cases. There are many societal factors, too, that affect the risk of dementia. Lower levels of education are linked to higher risks. Many parts of India have had limited access to formal education, especially for older generations. This increases the cognitive vulnerability of the elderly. Cognitive reserve (the brain's ability to cope with damage) tends to be higher in people with more education, which helps delay dementia onset. Conditions such as hypertension, diabetes, obesity and high cholesterol, which are widely prevalent in India, increase dementia risk. Cardiovascular health directly affects brain health, as poor blood flow can damage brain cells over time. Urbanisation and lifestyle changes have led to higher rates of these conditions, exacerbating the risk in India. Sedentary lifestyles, smoking, and unhealthy diets (rich in processed foods) are risk factors for dementia. India has seen significant lifestyle shifts over recent decades, particularly in urban areas, increasing exposure to these risks. Conversely, traditional Indian diets, rich in plant-based foods and spices, may offer some protective effects, but these benefits are declining with lifestyle changes. Social isolation, loneliness and mental health conditions like depression are common among the elderly in India. Cultural shifts have led to more elderly individuals living alone or in nuclear families rather than extended families, reducing social engagement. Air pollution is a growing concern in India, especially in cities. Emerging research suggests that long-term exposure to air pollution can increase the risk of cognitive impairment and dementia.
A monthly column that explores themes on nature, nurture and neighbourhood in the shaping of form and function.
In addition to lifestyle impact, genetics plays a significant role in dementia risk, particularly for Alzheimer's disease. In India, studies on genetic risk factors are emerging, but certain genetic changes associated with Alzheimer's, and other neurodegenerative conditions, are also seen in the Indian population. For instance, the APOE ε4 or apolipoprotein E epsilon 4 is a genetic variant of the APOE gene that is strongly associated with an increased risk of developing Alzheimer's. The APOE ε4 allele is characterised by two specific changes in the gene, which alter the corresponding protein's function and increase Alzheimer's risk. APOE ε4 is less efficient at clearing amyloid-beta, a protein that can accumulate into plaques, which are characteristic of Alzheimer's disease. This is why individuals with one or two copies of the APOE ε4 allele have a higher risk of developing Alzheimer's compared to those with the ε2 or ε3 variants.
Intensive research is still ongoing to determine the prevalence of these genes in different Indian subpopulations. Poor healthcare access and a lack of awareness about dementia also exacerbate the issue, leading to late diagnosis and inadequate care. India's healthcare system is still developing in terms of geriatrics and dementia care. Many elderly people still lack access to early diagnosis or proper management of dementia, which worsens outcomes. Cultural stigma around mental health also plays a role, as many communities may not recognise the signs of dementia or seek help due to the stigma associated with cognitive decline.
FOCUS ON ALZHEIMER'S
While dementia encompasses a group of neurodegenerative disorders characterised by a decline in cognitive functions severe enough to interfere with daily life, Alzheimer's disease is the most common form of dementia, accounting for 60-80% of the cases. This translates to around 7-8 million people, out of the total 12 million at risk for dementia, in our present population. This is not a small number. Alzheimer's disease is characterised by a gradual onset and progressive decline in cognitive functions, particularly memory, language and visuospatial skills. Memory loss especially affects recent events and learning new information. Cognitive decline is seen by the impairment in reasoning, problem-solving and executive functions. Mood swings, apathy and social withdrawal are also significant changes that are easily seen.
The WHO estimates that around 5-8% of people over the age of 60 are affected by dementia at any given time; the risk increases as people age.
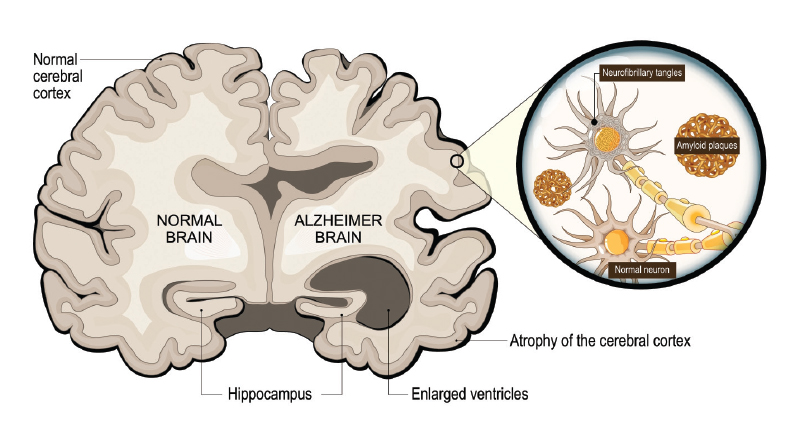
Alzheimer's disease is most likely caused by harmful changes in the brain, two of which have been identified in the research over decades. These changes relate to amyloid plaques and tau tangles. In Alzheimer's, the amyloid-beta protein clumps together outside brain cells, forming sticky plaques (the amyloid hypothesis). These plaques build up between brain cells and block the way brain cells talk to each other, disrupting their normal function. Over time, this damage affects how the brain processes information, leading to problems with memory and thinking. Also, inside brain cells, a protein called tau normally helps keep the structure of the cells stable. However, in Alzheimer's, tau becomes abnormally altered (through a process called hyperphosphorylation), causing it to tangle up. These tangled proteins disrupt the cell's internal structure, leading to the collapse of the "tracks" that transport nutrients and signals inside the cell. This eventually causes the brain cells to die.
One of the first areas affected in Alzheimer's is the hippocampus, a part of the brain involved in forming new memories. The entorhinal cortex, an area of the brain in the medial temporal lobe, which is important for processing and storing information, is also impacted. As a result, people with Alzheimer's often experience memory loss and difficulty learning new things in the early stages of the disease. The selective vulnerability of the hippocampus and medial temporal lobe in the early stages of many forms of dementia explains the predominant impairment of short-term memory. Long-term memories, stored across widespread cortical areas, remain relatively intact until later stages, reflecting the disease's progression and regional specificity. Alzheimer's disease is thus marked by harmful protein build-ups inside and outside brain cells, which damage the brain's communication networks and lead to the death of brain cells, especially in areas responsible for memory.
SETBACKS IN RESEARCH
As research was surging ahead, the field has been rocked by scandals. The recent detection of fraudulent publications has dealt a significant blow to Alzheimer's research, particularly regarding the widely studied amyloid hypothesis. This hypothesis posits that the build-up of amyloid-beta plaques in the brain is a central cause of Alzheimer's disease. However, recent investigations have uncovered manipulated images in key scientific papers, raising concerns about the integrity of foundational research supporting this hypothesis.
The fraudulent data, which were part of influential studies published over the past 15 years, involved alterations to images of amyloid-beta plaques in brain tissue. These manipulations have cast doubt on some specific findings, particularly around certain sub-types of amyloid-beta, which were thought to play a critical role in Alzheimer's progression. As a result, several clinical trials based on these findings may have been affected, leading to misallocated resources and potentially slowing progress in the search for effective treatments.

In an independent case, Eliezer Masliah, a prominent neuroscientist and former Director of the Division of Neuroscience at the National Institute on Aging, a division of the National Institutes of Health (NIH), was recently found to have falsified or manipulated images in over 100 research papers published between 1997 and 2023. These manipulated figures were reused across different studies, raising concerns about the validity of his work on neurodegenerative diseases like Alzheimer's and Parkinson's. Forensic analysis led to retractions and corrections in several papers, questioning the integrity of much of his research, which had influenced drug development. The NIH concluded its investigation in 2024, resulting in Masliah's removal from his leadership role.
Despite these setbacks, it is important to note that the amyloid hypothesis as a whole has not been fundamentally invalidated. The hypothesis is based on decades of research, much of which remains sound and is supported by independent studies. However, the revelations about data fraud underscore the complexity of Alzheimer's disease and the possibility that amyloid plaques may be just one of several contributing factors, rather than the sole cause.
NON-TRADITIONAL TESTS
A very interesting development that has gained prominence in recent years is the use of dried-blood spot analysis for molecular markers of dementia and other diseases. Much of this work has been led by Henrik Zetterberg and colleagues at the University of Gothenburg and University College London, as part of the UK Dementia Research Institute. Zetterberg and his team have been advancing the field of Alzheimer's disease diagnosis through the development of blood-based biomarkers, particularly using dried blood spot (DBS) sampling. In work published in Nature Aging in 2023, the team highlights how these biomarkers, including phosphorylated tau (p-tau), are revolutionising Alzheimer's detection. These biomarkers can now be measured accurately in plasma and dried blood, offering a less invasive, more accessible alternative to cerebrospinal fluid (CSF) analysis and PET scans traditionally used to diagnose Alzheimer's.
This approach holds promise for early detection, monitoring disease progression and even screening larger populations, which is particularly important for clinical trials and in regions with limited healthcare access. The research demonstrates that biomarkers such as p-tau217 in blood samples strongly correlate with Alzheimer's pathology, providing a cost-effective and scalable solution for diagnosing and tracking the disease.
This innovation could reduce reliance on costly procedures, making Alzheimer's diagnosis more accessible worldwide, and improve patient outcomes through earlier intervention and monitoring of disease progression. Researchers in India, too, are already working in this direction, promising the deployment of markers for easier and earlier detection, and even the potential discovery of new markers.
Also Read
Alzheimer's Association. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement., 19 (4), 700-789 (2023). bit.ly/2023-data
Selkoe, D. J., and Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 30 years. EMBO Mol. Med., 15 (5), e16301 (2023).
Hansson, O., Blennow, K., Zetterberg, H. et al. Blood biomarkers for Alzheimer’s disease in clinical practice and trials. Nature Aging, 3, 506-519 (2023). go.nature.com/3BABIrK
See also:
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH















