Bend it like a polymer
-
- from Shaastra :: vol 03 issue 08 :: Sep 2024
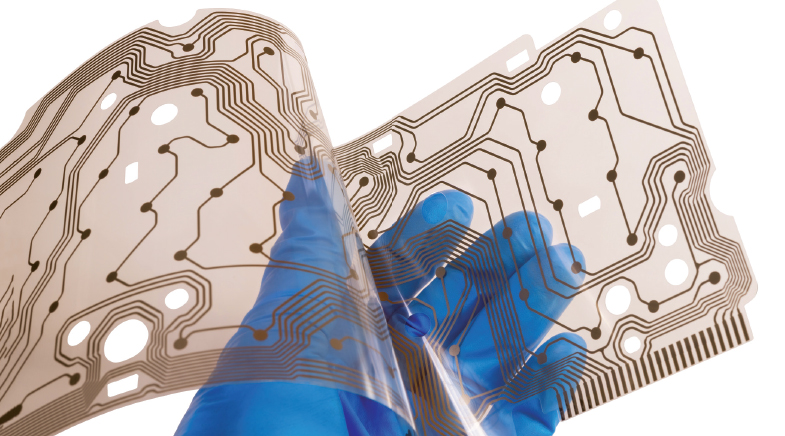
Scientists are boosting the conductivity and stability of organic semiconductors to create flexible sensors and bioelectronics.
For scientist Chi-Yuan Yang, organic chemistry is like toying with building blocks. "If you play with a variety of molecules, you may eventually make new matter, new polymers," says Yang. As an Assistant Professor at the Laboratory of Organic Electronics at Linköping University in Sweden, Yang has been researching conductive plastics, creating polymers that can act as an alternative to semiconductors made of silicon. While organic electronics has paved the way for flexible screens, biocompatible sensors, organic light-emitting diode screens, and sustainable power storage, they have lower conductivity and stability than inorganic semiconductors.
Along with Simone Fabiano, Principal Investigator at the lab, Yang has found a new way to improve the conductivity of organic semiconductors using light and oxygen from the natural environment. The main player here is doping — that is, increasing the conductivity of a semiconductor by adding impurities. The dopant can either remove electrons from the substrate in an oxidation reaction, creating holes (p-doping), or introduce electrons through a reduction reaction, creating a negative charge (n-doping). "Doping is... really the underpinning of all electronic devices. We won't be able to talk right now without the silicon in our devices being doped, and so the same applies to organic semiconductors," Fabiano says over a video call from Sweden.
Fabiano and Yang started working with groups in China and the U.S. on the catalytic doping of organic semiconductors. They first experimented with gold nanoparticles, but that was an expensive exercise. "Moreover, we still needed to activate the reactions by heating up the substrate. And most of the time we would lose the catalyst as it would either be deposited on the substrate or blend with the semiconductor. So we were wasting the catalyst," Fabiano explains.
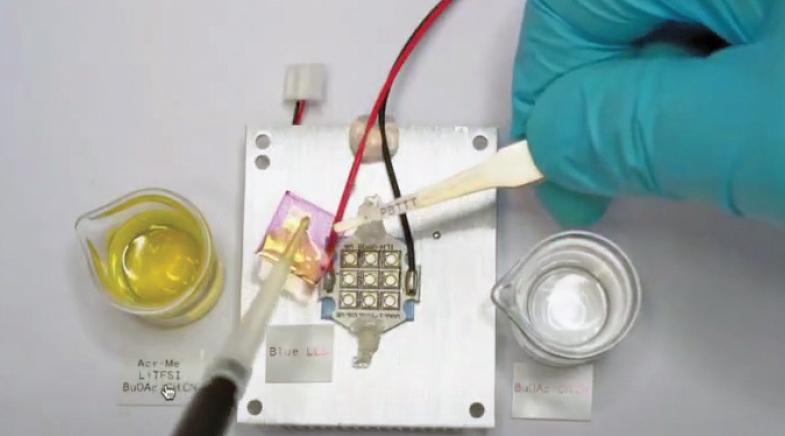
The team then began searching for a solution that involved activating the reaction from outside — using photocatalysis or light as an accelerant for doping. Yang dipped a polythiophene polymer in a photocatalytic solvent and shone a blue LED light on it. This made it possible for even weak dopants such as oxygen in the air — which would otherwise be unable to react — to increase the charge density of the semiconductor. "The advantage is that with this technique, you can dope both n-type and p-type semiconductors simultaneously to make a thermoelectric generator or a field effect transistor, for example," says Fabiano.
A study from Tianjin University in China proposes using vitamin C to stabilise n-type organic semiconductors by inhibiting the reaction of oxygen.
Traditionally, inorganic semiconductors such as silicon outperform organic polymers in terms of conductivity. This difference in charge mobility arises from their molecular structure: silicon is packed in a three-dimensional crystalline lattice, while polymer chains are one-dimensional with higher disorder. Doping in inorganic semiconductors happens in parts-per-million levels; to achieve that level of conductivity a much higher number of molecules has to be added.
For a "good level" of conductivity, the molecules needed have to be 5-10% of the polymer's weight, explains Satish Patil, Professor, Solid State and Structural Chemistry Unit, Indian Institute of Science (IISc), Bengaluru. "However, this also introduces a dead mass in the system and creates more disorder. So, you have to be clever in how you dope these materials. That is the challenge researchers in this field have been interested in," Patil adds.
After completing his post-doctoral work at the University of California in 2006, Patil returned to India to teach at IISc, where he set up a lab called the Organic Electronics Group. Patil points out that by training he is a polymer organic chemist, but he has also been interested in physics since his childhood. "What drove me to set up a lab like this was the opportunity to not only practise pure organic chemistry but also seek application of these molecules in optoelectronic devices. It is the interface of my favourite subjects." The lab works at creating photovoltaic cells, electrochemical energy storage devices, and organic field-effect transistors.
FLEXIBILITY AT LOWER COSTS
Patil stresses that organic electronics did not necessarily evolve to replace silicon, but to complement it. As polymers are solution-processable, they don't have to be manufactured at high temperatures, needed for obtaining crystalline silicon. This process lowers operational costs and facilitates roll-to-roll printing of these semiconductors on the substrate.
Solution-processable and printable polymers have a significant advantage: flexibility. "You can coat a thin film of the polymer on a flexible substrate, and it will be sustainable. Silicon is very rigid, and it's not as easy to grow silicon on a flexible substrate," says Patil. This kind of flexibility has paved the way for creating devices such as foldable and stretchable screens, compatible with wearable devices.
Fabiano and Yang started working on the catalytic doping of organic semiconductors and searched for a solution that involved activating the reaction from outside.
At the Organic Electronics Group, Patil and his team have been developing a new class of materials by modifying a DPP (diketopyrrolopyrrole) dye — used initially in the automobile industry for its stability under light — and tuning it for specific applications in organic electronics. The group created an ambipolar semiconductor from DPP with both holes and electrons, implying that it could conduct electricity in both directions and act as an n-type and a p-type semiconductor. For this material, they have also been researching dopants.
In p-type doping, the impurity takes electrons from the semiconductor atom's highest energy band — the valence band. However, physicists at the Cavendish Laboratory at the University of Cambridge, U.K., recently showed that they could not only empty out the valence band electrons but also remove electrons from one energy band deeper, creating higher levels of conductivity in polymers.
In principle, this may be done for any semiconductor. But in lattices such as silicon, removing electrons from deeper valence bands may cause a material to fall apart. "That's because the electrons that are binding it together are the same ones that you're removing," explains Ian Jacobs, one of the authors of the physicists' study published in Nature Materials (go.nature.com/3ySIhFl). "In organic semiconductors, however, the bonds holding the polymer together differ from the bonds conducting the charge. So you can remove that whole valence band and go further down the energy bands," he says.
FUTURE OF BIOELECTRONICS
The team at Cavendish Laboratory is interested in creating mixed ionic-electronic polymer conductors that can transport charges through ions and electrons. Mixed ionic-electronic conductors form the basis for organic electrochemical transistors that are increasingly finding use in biocompatible sensing devices.
Transistors act as gates that regulate the flow of a current and consist of both n-type and p-type semiconductors arranged in a certain fashion. For them to be truly effective, the two types should have conductivity on a par with each other. In organic electronics, however, one major drawback has been the lack of stable n-type semiconductors. These polymers react to moisture and oxygen in the air under ambient conditions and cause instability.
When Fabiano started his research a decade ago, his focus was getting the conductivity of the n-type to match that of the p-type polymers. In 2021, he and Yang co-founded a company, called n-ink, to develop polymer inks for printed organic electronics. The start-up manufactures organic batteries, supercapacitors, and solar cells that run specifically on n-type semiconductors. Recent research efforts worldwide have focused on improving the stability of n-type polymers. A study from Tianjin University in China proposes using vitamin C to stabilise n-type organic semiconductors by inhibiting the reaction of oxygen (go.nature.com/3X1ULm6).
With advancements in organic electrochemical transistors, Fabiano is excited about the potential of creating electronic devices that work more closely with human biology — "that speak the same language", as he puts it. Organic materials are soft and biocompatible, and can be interfaced with the brain. They could make neuromorphic devices that resemble and emulate synapses and, eventually, neural circuits in the human brain. "I think this is a good time also for starting up a company in the field of organic electronics," he says.
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH