Green is the new black
-
- from Shaastra :: vol 02 issue 02 :: Mar - Apr 2023

Chemists across the world have taken on the task of decarbonising the carbon-spewing chemical industry. Some new measures show promise.
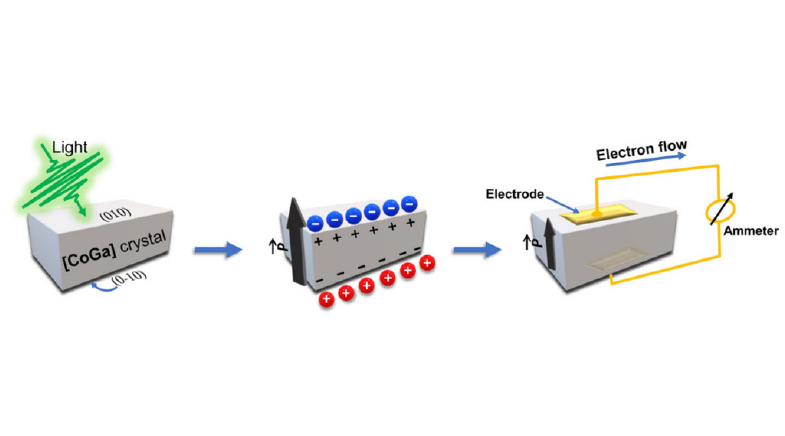
As a child, Karthish Manthiram was fascinated by the nature of electricity. He used to play with batteries, wires and bulbs, trying to form circuits and see which way the electrons would move and form currents. Now, as Professor of Chemistry and Chemical Engineering at the California Institute of Technology (Caltech), he plays in his office in the same vein, trying to coax electrons to move from one molecule to another, figuring out ways to do that without impacting the environment.
Electrons move from one atom to another during a chemical reaction. Sometimes atoms need to be brought close enough – by spending energy – to facilitate this electron movement. The Haber-Bosch process, for example, uses high pressure and temperature to manufacture ammonia from nitrogen and hydrogen. Because ammonia is a raw material for fertilizers and other valuable chemicals, this process improved the quality of life for billions of people. But not without a big impact on the environment.
Chemists are now developing electrochemical processes to manufacture industrial chemicals, with very low carbon footprint, using renewable energy as a source of electrons. "A long time ago, making it as cheap as possible is what they all cared about," says Manthiram. "A lot has changed 100 years since that process was discovered. Now we have to reinvent methods of making ammonia that is more environment-friendly."
Manthiram is among more than a dozen scientists trying to make ammonia manufacture environment-friendly, among other things. A century ago, the Haber-Bosch process was a game-changer for the world, by increasing food production and providing the world with cheap materials. Electrochemical synthesis of ammonia is set to be a game-changer on a similar scale, by expanding the production of ammonia without affecting the environment.
Manthiram loved chemistry, too, as a child. Even as he made and played with electric circuits, he also performed chemistry experiments at home, developing colloid emulsions and experimenting with acid-base reactions. This twin love, of electricity and chemistry, subconsciously influenced many choices that he made later in life. As a doctoral student and, later, as a researcher, he enjoyed synthesising molecules and developing electrocatalysts.
PAST ISSUES - Free to Read


Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH