Scientists use metal-organic frameworks to capture carbon
-
- from Shaastra :: vol 03 issue 08 :: Sep 2024
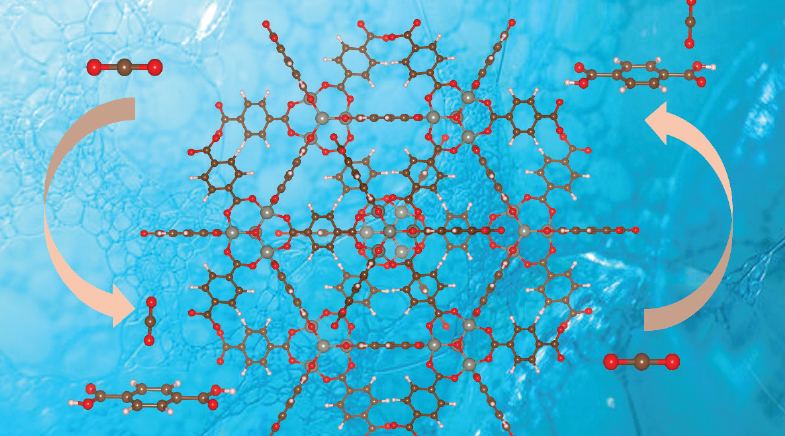
Physicists capture carbon dioxide with porous metal-organic frameworks to control emissions.
For almost three decades, metal-organic frameworks (MOFs) have been used for gas separation and storage. These organic polymers have metal nodes that link to form a rigid but porous 3D structure. Omar Yaghi at UC Berkeley pioneered the field of MOFs and covalent organic frameworks (COFs) in the late 1990s. Since then, researchers have been experimenting with different ways to utilise their porous structure for different purposes. "First, people focused on hydrogen capture, so that it could be stored and used as an alternative green fuel. Right now, one of the popular uses is developing a carbon capture system," says Srimanta Pakhira.
At the Indian Institute of Technology Indore, Pakhira is experimenting with using the pores in MOFs to capture carbon dioxide. Pakhira believes MOFs to be the next big thing in carbon capture and storage technology. Traditionally, MOFs contain metal nodes of copper or zinc, bridged together via ligands such as 1,4-benzenedicarboxylic acid (BDC). Together with his student Kahkasha Parveen, Pakhira aimed to increase the carbon dioxide absorption into the pores of MOFs (rsc.li/4deJ7uh).
"One of the ways to do that was to increase the negative binding enthalpy of the reaction, so that it would be more stable," says Pakhira. He replaced a few hydrogen atoms in the BDC with halogens like fluorine to increase the electronegativity. "Hydrogen is already saturated. But if you have fluorine in there, when CO2 molecules enter the pore, there will be greater interaction between the two," he says, referring to the weak van der Waals interactions that occur between two un-bonded molecules in proximity.
IIT Indore scientists are experimenting with using the pores in metal-organic frameworks to capture carbon dioxide.
Though Pakhira's investigations showed a higher negative binding enthalpy, it would not have been enough to capture carbon dioxide at a significant level. In the next step, he decorated the MOF with lithium, the lightest metal with a high electrochemical potential. "This enhanced the weak dispersion forces in the ligand, retaining any carbon dioxide molecule that comes in," he says. Once the CO2 has been captured inside the MOFs, it needs to be released and broken down to methanol via a catalytic reaction.
Pakhira and Parveen have tested the MOF at the quantum level for stability. To get it to industrial standards, they had to make sure it remains stable and efficient at ambient conditions as well. They are now building a 3D structure of the MOF on a larger scale. "At a laboratory level, with standard temperature and pressure, we can absorb up to 6 cubic centimetres of CO2 per gram of the MOF," says Pakhira, adding that they could imagine using it in a factory setting to control emissions at the source. "For it to develop to that stage, I would give it 5-10 years," he adds.
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH














