A superfine biosensor
-
- from Shaastra :: vol 02 issue 05 :: Sep - Oct 2023
Swiss researchers have created a biosensor to detect
neurodegenerative diseases and track their advancement.
A diagnosis of Parkinson's or Alzheimer's disease is worrisome in itself. However, to figure out a treatment plan, the doctor needs to determine the stage of disease progression. And that is a tall order.
Researchers at the Swiss Federal Institute of Technology in Lausanne (EPFL) have created a gold nanostructure-based biosensor to detect neurodegenerative diseases and track their advancement (bit.ly/ImmunoSEIRA).
Proteins in unaggregated or monomer forms are needed for the brain to function. In pathological cases, though, some proteins misfold into aggregates: α-synuclein misfolds in Parkinson's disease, and β-amyloid in Alzheimer's. As the disease progresses, these proteins aggregate into different structures called oligomers (small aggregates) or fibrils (thread-like aggregates). The quantity of oligomers and fibrils indicates the degree of disease progression.
Researchers argue that differential diagnosis and testing levels of protein biomarkers in bodily fluids lack sensitivity to protein aggregate structure, providing limited insights into disease progression.
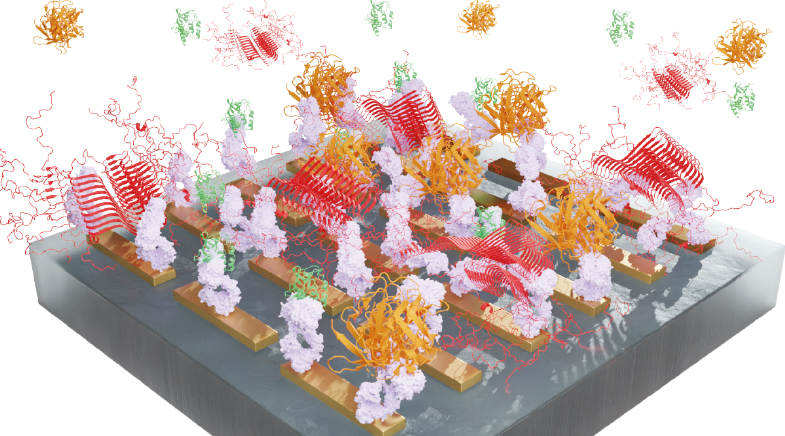
The optofluidic ImmunoSEIRA sensor integrates infrared light to test bodily fluids such as the cerebrospinal fluid and blood.
Deepthy Kavungal, the study's first author and Doctoral Assistant at EPFL's Bionanophotonic Systems Laboratory, advocates infrared spectroscopy. Every protein absorbs a specific portion of the incoming infrared light, says Kavungal. Analysing transmitted light for the absorbed portion can identify both the protein and its structure.
The optofluidic ImmunoSEIRA (Surface-enhanced InfraRed Absorption) biosensor, which Kavungal and colleagues developed, integrates infrared light for testing bodily fluids such as the cerebrospinal fluid (CSF) and blood. It employs an intelligent array of gold nanostructures that makes tiny hotspots for infrared light where the interaction of light with proteins is amplified. This guarantees that the sensor needs only a few drops of the sample to function.
Since the surface of gold nanostructures is coated with antibodies, which can selectively bind to specific proteins present in bodily fluids, only biomarker proteins attach to the nanostructures when a fluid passes through. Although the biosensor detects structural variations in biomarkers, it doesn't independently quantify them for information on disease progression. To address this, researchers harnessed artificial intelligence.
They input biosensor-generated data with known fibril concentrations into a Deep Neural Network (DNN), training it to recognise absorbance data patterns and predict unknown concentrations. The DNN model achieved high accuracy in quantifying α-synuclein protein fibril structures, a Parkinson's disease biomarker, the study demonstrates.
The medical community's aversion to new technology is the main challenge in taking the biosensor to the clinics, says Kavungal. "We took healthy CSF to validate the biosensor so that it can go to the clinical level, and we also tested this (biosensor) with the blood plasma."
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH