Keys to a puzzle
-
- from Shaastra :: vol 04 issue 02 :: Mar 2025
A 3D map of a 'promiscuous' protein opens a pathway to new drugs.
Since its discovery nearly three decades ago, a protein that protrudes from the surface of human cells has acquired the labels of both "a promiscuous molecule" and a "silent enabler" of disease. The protein, known as CXCR2, regulates immune responses, but also drives inflammatory conditions such as asthma, the growth and spread of some cancers, and certain chronic health disorders.
Given its role in multiple diseases, CXCR2 stands out as a high-priority target for drug discovery. Scientists want to design molecules that can block the protein's activity and stall the disease process. However, to do this, they need a precise 3D map of CXCR2 – its atomic structure and how it interacts with other molecules. Until now, the lack of such insights has left drug designers flying blind.
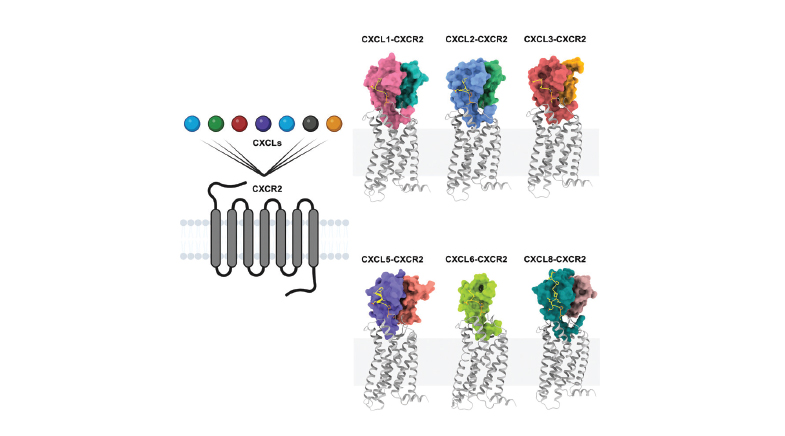
Now, structural biologist Arun Shukla at the Indian Institute of Technology (IIT) Kanpur and his colleagues have mapped CXCR2's atomic structure, revealing how its 360 amino acids – the building blocks of all proteins – fold into a complex helical architecture. Their research has also uncovered a previously unexplored site on the protein where two amino acids, arginine and aspartic acid, sitting right next to each other, form a distinct pocket-like feature. Their findings, published recently in the journal Molecular Cell (bit.ly/molecular-chemokine), suggest that this site is crucial to CXCR2's disease-driving function and could be a promising focus for drug design.
"The target is in our sights," Shukla, Professor in the Department of Biological Sciences and Bioengineering at IIT Kanpur, who supervised the study, told Shaastra. "Imagine the site of the two amino acids as a lock. We now have a glimpse of its insides and we need to design the right keys."
CXCR2 belongs to a family of receptor proteins that sit on the surface of cells, acting as docking stations for signalling molecules. These signals help direct immune system cells to sites of infection or injury and regulate other housekeeping functions in the body.
A typical lock works with only one key. However, CXCR2 is different: it binds to seven distinct signalling molecules, each with a unique structure, each triggering different cellular responses. In 2019, scientists at the University of Notre Dame in the U.S. showed that when CXCR2 binds with a molecule called CXCL5, it drives the proliferation and spread of breast cancer cells to bones and bone marrow. The ability to interact with multiple partners has earned CXCR2 its 'promiscuous' tag.
In 2019, scientists at the ShanghaiTech University in China were the first to describe the atomic structure of CXCR2 in a state bound to a signalling molecule called CXCL8, which directs a type of white blood cells called neutrophils to sites of infection or inflammation. They had used the cryogenic electron microscopy (cryo-EM) technique, in which a protein is frozen to temperatures of –180°C. An electron beam is used to generate thousands of images of the protein from multiple angles, and the images are processed to reconstruct a high-resolution 3D structure.
The IIT Kanpur researchers, with collaborators at The University of Tokyo and the Luxembourg Institute of Health, leveraged cryo-EM to gain new structural insights into CXCR2. Their imaging studies have revealed that a common amino acid triplet — arginine, aspartic acid, and glutamic acid — found in all seven signalling molecules binds exclusively to the pocket formed by CXCR2's two key amino acids. This interaction provides a molecular explanation for CXCR2's ability to engage multiple partners.
"This cryo-EM-driven advance in understanding CXCR2's molecular interactions could help accelerate drug discovery efforts," says biochemist Krishna Rajarathnam, Professor at The University of Texas Medical Branch, who was not associated with the new study but has done research on CXCR2's role in physiology.
"We've had a fuzzy picture until now and efforts to develop CXCR2-blocking drugs have not translated into a commercial product," Rajarathnam told Shaastra. "The structural snapshots from Shukla's team provide a much sharper picture that could lead to a clearer strategy to target this important receptor."
Shukla and his colleagues have now begun looking for molecules that could serve as a novel therapeutic candidate, using large chemical databases and computer simulations to try and identify molecules that will bind to the two-amino acid pocket.
"The structure of a candidate therapeutic molecule may need to be tweaked to ensure that CXCR2 has (a) greater affinity for the drug than for its natural signalling molecules in the body," said Manisankar Ganguly, a post-doctoral scientist and a member of the research team. The researchers caution that any promising candidates would need to be evaluated through lab studies, animal trials, and human clinical trials to establish its safety and efficacy.
G.S. Mudur is a science journalist.
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH