New tech tools are targeting mosquitoes
-
- from Shaastra :: vol 03 issue 07 :: Aug 2024

Scientists are working with novel technological tools to combat the mosquito menace.
The buzz and the bite of mosquitoes have been immortalised in poetry and prose down the ages; they have also inspired scientists to explore quirky ways to prevent the spread of malaria (bit.ly/mosquito-chicken). As journalist Sonia Shah wrote more than a decade ago in her book, The Fever, "We've had plenty of time – our entire evolutionary history, in fact – to adapt to malaria, and it to us. Or, at least, to devise tools and strategies to blunt its appetite. And yet... it remains essentially wild and untamed, despite its great antiquity."
To this day, malaria continues to claim thousands of lives every year. If anything, the umbrella of mosquito-borne diseases has only grown. The Anopheles mosquito, which ferries the malaria parasite from person to person, is resurfacing in places where it was wiped out years ago. Another mosquito, Aedes aegypti, the vector for dengue, chikungunya and Zika, is the "new contender for most lethal animal" (bit.ly/lethalanimal). Conventional methods of mosquito control are falling short, and a global effort to create innovative and effective solutions to the mosquito problem is underway.
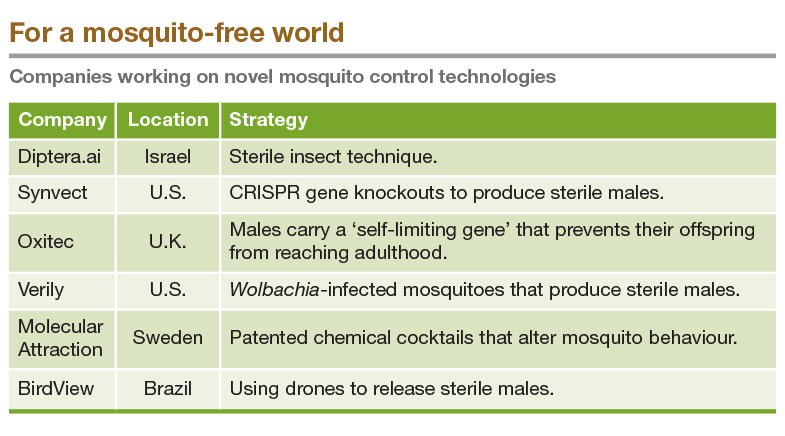
Some researchers are leaning into tools of genetics and molecular biology to stop the mosquitoes. In a June 2024 paper in PNAS (bit.ly/precisionSIT), researchers report a method that uses the CRISPR Cas9 system to curb the Anopheles gambiae mosquito, which spreads malaria in Africa. Using the CRISPR Cas9 system, the researchers made precise modifications in the mosquito DNA that make the male mosquitoes sterile. When released, these sterile males would mate with females, but no offspring would be produced. "If you can release sterile males en masse, it acts as a chemical-free, species-specific insecticide," says Andrea Smidler, one of the authors of the study and Postdoctoral Researcher at the University of California San Diego.
To produce these sterile males, the researchers first developed two transgenic/genetically modified lines of An. gambiae: one that produces the Cas9 enzyme and another that produces the guide RNA (a fragment of RNA that identifies the regions on the DNA for the Cas9 enzyme to cut).
The Anopheles mosquito, which ferries the malaria parasite from person to person, is resurfacing in places where it was wiped out years ago.
On their own, both the transgenic lines have normal fertility, and produce 50% males and 50% females. However, when the two lines are crossed with each other, the hybrid offspring get both, the Cas9 and the guide RNA. The two can then work together to modify the mosquito DNA at precise locations. In this case, the Cas9 targets the genes critical for sperm development and stops sperm production. It also acts on another gene critical for female development that stops females from being produced. So, the hybrid offspring consist exclusively of sterile males. "It is a high-precision method," says Smidler. The researchers call this method precision-guided sterile insect technique (pgSIT). In the lab, the researchers got over 99.5% male sterility and 99.9% female lethality, but it has not been tested in real-world situations.
Sterility has been used as a tool to control insect and pest populations for decades. The common method to induce sterility has been to irradiate insects with gamma rays or X-rays. But this doesn't work on An. gambiae, which is why the researchers opted for a genetics-based tool.
INHOSPITABLE MOSQUITOES
In another strategy to control malaria transmission, researchers at Transmission Zero, a mosquito research programme with researchers from Tanzania and the U.K., are making mosquitoes inhospitable for the malaria parasite. "We have made a very small change in the mosquito that makes it resistant to malaria," says Alexander Bailey, Communications Coordinator at Transmission Zero.
The malaria parasite Plasmodium uses the mosquito as a pit stop where it completes a part of its life cycle. The male and female gametes of the Plasmodium develop inside the mosquito mid-gut, which then combine to form the zygote. Through a series of steps, the zygote then develops into sporozoites that travel to the salivary glands, ready to be injected into the blood of the person the mosquito bites.
The researchers at Transmission Zero have come up with a way to interfere with Plasmodium's growth within the mosquito and prevent it from expanding its population. They have introduced a genetic tweak in the An. gambiae mosquito, which enables it to produce two anti-microbial peptides: magainin 2 and melittin. These anti-microbial peptides prevent the growth of malarial parasites, P. falciparum and P. berghei, inside the mosquito's mid-gut. Deprived of the conducive environment within the mosquito's mid-gut, the parasite can no longer complete its life cycle.
Additionally, these mosquitoes can cause a gene drive, which means that the resistance can spread from them to other mosquitoes in the environment. Unlike the sterility-based methods that work by suppressing a population of mosquitoes, this method works by population modification. A suitably modified mosquito is released, which can then spread the modification to other mosquitoes.
BACTERIA-INFECTED MOSQUITOES
In another strategy that has gained ground in the last decade, researchers use a bacterium named Wolbachia to bring down mosquito numbers. Wolbachia naturally infects many insect species, including mosquitoes. Interestingly, Wolbachia-infected female mosquitoes have a reduced ability to transmit viruses like dengue and Zika. When Wolbachia-infected males mate with regular healthy females, their eggs don't hatch. When infected mosquitoes are released in an area over a period of time, Wolbachia infection spreads in the mosquito community, resulting in lower rates of disease transmission, fewer mosquito eggs hatched, and eventually a reduced mosquito population.
TIGS Director Rakesh Mishra reckons that with urbanisation, many mosquito species, which earlier infested only densely populated cities, are spreading to rural areas.
Since 2011, the not-for-profit World Mosquito Program (WMP) has used this method to bring down the numbers of Ae. aegypti mosquito and, consequently, dengue transmission in a number of places around the world, including North Queensland in Australia, Rio de Janeiro in Brazil, Medellin in Colombia, and Yogyakarta in Indonesia. Most recent of these was a three-year-long randomised controlled trial in Yogyakarta (bit.ly/dengue-study). Wolbachia-carrying Ae. aegypti mosquitoes were released every two weeks over seven months in 2016-17 in a 5-km2 area. A 3-km2 area on the other side of the city was designated as the untreated, control area. At the end of the trial, the researchers saw a 77% reduction in dengue incidence and an 86% reduction in dengue hospitalisations in Wolbachia-treated areas compared with untreated areas.
A specific strain of Wolbachia called wMel was used in WMP's trials. There have been instances where this strain has not performed as expected. An April 2024 article in The Lancet Microbe (bit.ly/imperfect_wolbachia) says: "The wMel strain was unable to penetrate local mosquitoes in Tri Nguyen, Viet Nam, and resulted only with intermediate levels of introgression in Rio de Janeiro, Brazil, despite the release of over 70 million insects." Why the wMel strain's performance varies from place to place is not fully understood yet. But some studies have shown high temperature could be a factor.
Using the right strain of Wolbachia is important. At the Tata Institute for Genetics and Society (TIGS), Bengaluru, researchers are working on developing Wolbachia-infected mosquitoes with an aim to control the spread of mosquito-borne diseases. They want to use local Wolbachia strains and are hunting for them in local insects like Exorista sorbillans, Drosophila sp., Aedes albopictus, Culex quinquefasciatus and Trichogramma spp. "The key attributes that we look for is the resilience of the Wolbachia to higher temperatures, its ubiquitous distribution throughout different somatic and reproductive tissues, relative density, co-dominance during multiple infections and ability to induce reproductive anomalies in the host insects," says Rakesh Mishra, Director of TIGS. He is hopeful of releasing the Wolbachia mosquitoes in a few years.
The impact and footprint of mosquitoes in India is changing fast. "There has been a notable change in the mosquito larval habitats. Aedes mosquito larvae are expanding their niche and can be seen coexisting with Culex larvae in brackish water habitats. Due to urbanisation of villages, many anthropophilic mosquito species, which were restricted to densely populated cities, are expanding their range to rural areas," says Mishra.
Current solutions for mosquito control are no longer enough. Despite use of insecticides, mosquito nets and repellents, and the removal of stagnant water, cases of mosquito-borne diseases are high. Scientists reckon that it is now time to use novel genetic tools, which are starting to create a buzz.
See also:
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH