Pore-forming to the fore
-
- from Shaastra :: vol 04 issue 10 :: Nov 2025
Nanopore technology comes to the aid of biomedical applications.
Nanopore technology, which promises to help researchers understand and detect diseases, has taken a step closer to biomedical applications, potentially even leading to cancer therapies. Nanopores are nanometer-sized holes formed naturally by proteins on cell surfaces to allow biomolecules or ions to pass through. Scientists have found a way to synthesise them, harnessing such proteins naturally present or artificially creating them.
The technology has been widely used in DNA and RNA sequencing. For instance, Oxford Nanopore Technologies (ONT), which pioneered the technology for DNA sequencing in 2005, is today a 1,300-strong company with a net worth of $2 billion. Many other companies are also in the field. The technology, however, has yet to become a tool at the pathologist's disposal.
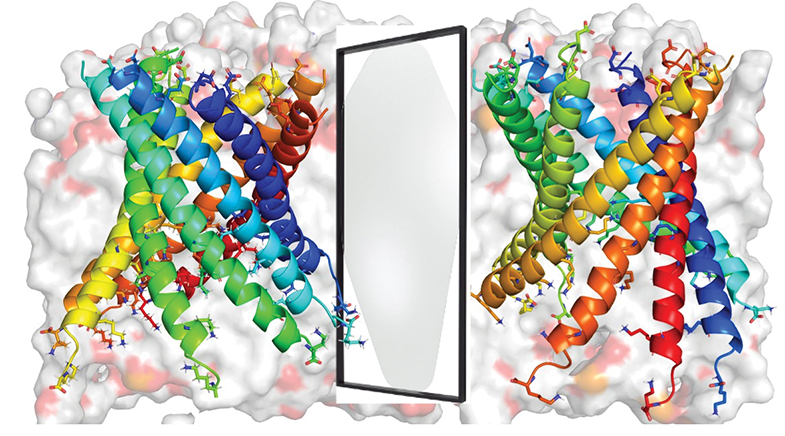
Now, Kozhinjampara R. Mahendran, formerly a postdoc student of Hagan Bayley, Professor of Chemical Biology at the University of Oxford, whose pioneering work led to the founding of ONT, has made a breakthrough with other researchers. Mahendran, a senior scientist at the Thiruvananthapuram-based Rajiv Gandhi Centre for Biotechnology (RGCB), and his collaborators have fabricated and characterised a fully functional nanopore from D-amino acids, which are mirror-image forms of naturally found L-amino acid proteins. Proteins in nature are mostly built from L-amino acids, with their D-amino acid counterparts having minimal roles to play.
The proteins in consideration are a class of membrane proteins that form channels on bacterial cell walls, mitochondria or chloroplasts. These channel proteins perform diverse tasks — transporting specific molecules and regulating the flow of ions — and are key to maintaining homeostasis (or equilibrium) in cells.
While it is an extremely challenging process, building pore-forming proteins from D-amino acids has several advantages. For one, they will not be degraded by proteases, enzymes that break down proteins and peptides, and will thus provide stable and longer-lasting nanopores that can be used for diverse applications in biotechnology and medicine.
PAST ISSUES - Free to Read


Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH














