It's not just a vaccine
-
- from Shaastra :: vol 01 issue 04 :: Jul - Aug 2022

mRNA also lends itself to therapeutics, but the technology's flaws need to be fixed.
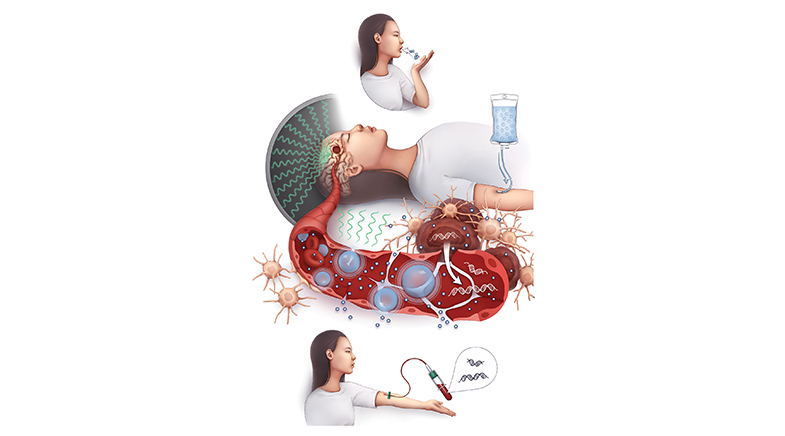
Treating cancer is expensive business. Consider monoclonal antibodies (MAB) — biological drugs that trigger the immune system to kill cancer cells by binding to certain targets such as proteins. Their complex development and manufacturing process is a key driver of cost, which often runs into lakhs of rupees.
For over a decade, scientists have experimented with a promising new technology – messenger Ribonucleic Acid (mRNA) – to address this. mRNA eventually earned its spurs in the pandemic with the two COVID-19 vaccines from U.S. companies Moderna and Pfizer/BioNTech. Applied to MAB therapeutics, the technology could potentially cut the cost per dose to a fraction of what it is now, says Anand Khedkar, co-founder of Sekkei Bio, a Bengaluru start-up.
Sekkei is among a raft of companies globally – from biotech start-ups to large drug makers – investing in mRNA’s many possibilities, including MAB therapeutics. Sekkei claims to have developed its own mRNA platform with studies ongoing to adapt it to MABs.
Votaries of mRNA believe that the vaccines are merely a preview to its myriad possible uses in cell reprogramming, gene editing and therapeutic vaccines to treat — and not prevent — disease. "mRNA is brilliant technology and it would be a waste to limit its use to only COVID vaccines," says Dhananjay Bakhle, a physician heading medical research for a major Indian drug maker.
However, the world is yet to see any mRNA therapeutics. The five mRNA products approved in the U.S., India and South Korea are all COVID-19 vaccines.
While mRNA has fascinated scientists for decades, attempts are still being made to address the shortcomings of first-generation mRNA.
In India, too, interest in mRNA has mainly come in from vaccine companies, says N. Madhusudhana Rao, CEO of Hyderabad’s Atal Incubation Centre — Centre for Cellular & Molecular Biology (AIC-CCMB). AIC-CCMB has designed a linear mRNA COVID-19 vaccine that uses Moderna’s product as a reference. The Indian Institute of Science, Bengaluru, is also working on an mRNA COVID-19 vaccine. The only approved mRNA vaccine in India, from Gennova Biopharmaceuticals, uses technology licensed from U.S. company HDT Bio.
PLUSES AND MINUSES
While mRNA has fascinated scientists for decades, attempts to address the shortcomings of the first-generation mRNA vaccines are still ongoing. Also, like any new technology, there are safety concerns.
mRNA has a slew of advantages. MABs are produced in large bioreactors on acres of land with multi-step pre-production, manufacturing and purification processes. mRNA is quick and less capital-intensive, capable of being made in a 1,000-sq-ft site using microfluidic equipment, says AIC-CCMB's Rao. The doses are smaller, which means higher manufacturing productivity. All mRNA vaccines are dosed in micrograms with a new generation self-amplifying vaccine effective at 5mcg (see box: 'More on mRNA').
There is a downside, too. mRNA is fragile and can be broken down by enzymes called endonucleases, the "beat cops" that view it "as loose pieces of information in the blood and extra-cellular space that could wreak havoc in the body", Bakhle says. This impacts efficacy. Its fragility also means that the first-gen COVID-19 vaccines have a relatively lower shelf life. They are formulated in lipids or fat shells that call for expensive storage at sub-zero temperatures.
Second, lab-made mRNA is recognised by receptors on and inside the cell, potentially leading to an undesired immune response. Writing in the molecular biosciences magazine The Biochemist, Anne Blakney, an mRNA researcher and Assistant Professor at the University of British Columbia, explains that this is a result of "innate sensing and detection of foreign RNA that humans have evolved to fight infections from RNA viruses". As a result, potentially less of the protein or antigen that the mRNA is designed for is expressed.
This could also lead to unacceptable side effects. For instance, a report in the December 2021 issue of Future Virology observes that an impurity called double-strand mRNA — a "strong inducer of immune-inflammatory reactions" — could be "hypothetically suspected" to trigger myocarditis, a rare side effect of the linear mRNA COVID-19 vaccines. The immunogenicity of lab-made mRNA "is the primary issue hampering the development of mRNA as a pharmaceutical", says a 2019 paper titled ‘How mRNA therapeutics are entering the monoclonal antibody field' in the Journal of Translational Medicine.
Third, vaccine mRNA lasts just a couple of days in the body. An mRNA drug therapy needs to last longer to be effective.
WORK IN PROGRESS
Scientists have modified certain nucleotides in linear mRNA vaccines to partially go around innate sensing. However, as Blakney writes, "the final frontier of the future of mRNA vaccines is minimising the dose required" as a lower dose could be potentially less immunogenic.
Sekkei's self-amplifying mRNA (saRNA) is designed to make copies of itself. Bakhle likens it to "creating a Xerox machine (for the mRNA) inside the cell". saRNA could potentially express more protein — which is vital for therapeutics — and its effective dose could be lower. Sekkei scientists are also working on circular or closed-loop mRNA (circRNA) to increase half-life — or how long it takes to break down — with similar objectives.
circRNA is also expected to be stable at higher temperatures. To test this, Sekkei will compare its circRNA with linear mRNA on the basis and duration of expression, shelf life and temperature stability.
An important component of a finished mRNA product is its delivery vehicle. The COVID-19 vaccines use lipid nanoparticles (LNPs) — a formulation system that encases mRNA in fat shells to protect them from degradation. The new-generation saRNA COVID-19 vaccines can be stored at room temperature or in a medical refrigerator because of advances in LNP formulations such as freeze-drying.
As LNPs can pose safety hurdles of their own, Sekkei has a research tie-up with a Canadian company, whose LNPs "have been extensively characterised and tested in humans for safety and efficacy", says Khedkar. He did not disclose the name of the company, citing confidentiality.
saRNA comes with its own set of challenges. "Linear mRNA versus saRNA is like a small coil versus a long coil which has to be packed like a yarn into the lipid particle," Bakhle says. The length of the mRNA, already a long molecule, could treble, leading to formulation challenges.
The new-generation saRNA COVID-19 vaccines can be stored at room temperature or in a medical refrigerator because of advances such as freeze-drying.
mRNA companies including Sekkei are experimenting with the design of saRNA — such as cis-acting and trans-acting mRNA (see box: 'More on mRNA' )— to balance ease of design and formulation with targeted delivery of the mRNA and its amplifiers into the same cell. Purification of saRNA may also pose a challenge as the equipment needed may be non-standard, hence not easily available, Bakhle says.
In MAB therapeutics specifically, both key sub-units of the MAB, the so-called heavy and light chain of polypeptides, have to be expressed and oxidised to form a complete MAB. These chains, of differing lengths and sequences, are determinants of its specificity (such as the antigen-antibody fit). "If this works, it would be a very big step forward to progressing MAB therapy to small footprint manufacturing," Khedkar says.
NOT ROCKET SCIENCE
Anything new takes time, Rao of AIC-CCMB points out. Consider the in-vitro transcription kits comprising reagents and enzymes that are core to mRNA manufacturing. These are imported and of variable quality. "It takes at least a month to come by; then if something doesn't work, you have to wait another month for the next one," he says.
There is a variety of microfluidic equipment to choose from. But Rao adds that the providers "won't tell you which works better". This requires poring over published literature and making a calculated guess. "It is not rocket science but a matter of intention and investment."

In spite of the challenges, Rao believes it is a technology that should be invested in. "The pandemic has taught us that we should be quick on our feet. mRNA has that advantage: it is quick and flexible," he says. An evolving technology also presents the advantage of India not being too far behind the West. "We are all learning together," says Khedkar. "That makes it a level playing field."
MORE ON mRNA
What is mRNA?
- A single strand of nucleotides in the human cell.
- A how-to guide for cells to produce proteins involved in bodily functions.
- Synthetic mRNA is made in the lab from a DNA template in a process called in-vitro transcription (IVT).
- Unlike conventional biologics manufacturing, IVT uses reagents and enzymes but no cells.
How does lab-made mRNA work?
- When delivered into the cell, mRNA is 'translated' or read by ribosomes to 'express' or make proteins.
- It is encased in fat bubbles or lipid nanoparticles that protect it from degradation.
What are the types of mRNA?
- Linear mRNA is a single strand of nucleotides with two free ends.
- Circular mRNA is made when the ends are linked.
- Self-amplifying mRNA codes four additional non-structural proteins, or replicase, from an alphavirus to help make copies of the mRNA. saRNA can be cis-acting or trans-acting.
- In cis-acting mRNA, the coded mRNA and the replicase are on the same construct. The size creates challenges of design and formulation.
- In trans-acting mRNA, two separate mRNA constructs are coded for the protein and the replicase but delivered together. The challenge is to deliver both to the same cell.
What are the benefits of mRNA?
- Doses are smaller. The Pfizer COVID-19 vaccine is 30 micrograms (mcg) per dose. A saRNA COVID-19 vaccine from U.S.-based Arcturus Therapeutics is 5mcg per dose.
- It is rapidly turned around. It could take three months from design to a scaled-up process where an MAB could take 24. The absence of cells in IVT reduces complexity.
- Enhanced manufacturing productivity.
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH