Borophane, the new ‘wonder material’, is here
-
- from Shaastra :: vol 01 edition 01 :: May - Jun 2021
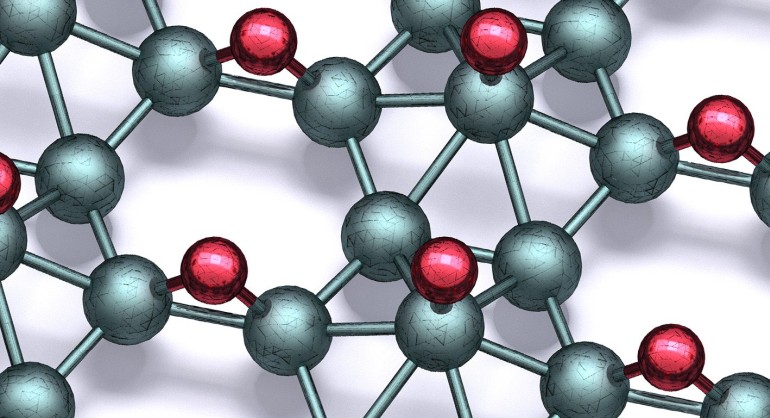
MOVE OVER graphene: borophane, considered a ‘wonder material’ of sorts, is here, and could potentially revolutionise batteries, electronics, sensors, photovoltaics and quantum computing. Created by bonding atomic hydrogen with a single-atom-thick sheet of boron, borophane is not just stable at standard temperatures and air pressures, but also has high mechanical strength, flexibility and superconducting properties.
Before borophane, there was borophene, an analogue of graphene, which was once hailed as a wonder material. The quest for more graphenelike two-dimensional materials prompted physicists to predict in the 1990s that boron atoms, too, can form a monolayer through computer simulations. However, it was synthesised only in 2015.
Studies have shown borophene to be stronger, lighter and more flexible than graphene. But borophene has a fundamental problem: it can only exist inside of an ultrahigh vacuum chamber. And this limited its practical use outside the lab. Now, a team of materials researchers led by Mark Hersam at Northwestern University in Illinois has overcome this problem by depositing atomic hydrogen onto borophene’s surface to develop borophane.
Among Hersam’s collaborators is Venkat Surya Chaitanya Kolluru, a B.Tech from the Indian Institute of Technology (Indian School of Mines) Dhanbad, and a graduate computational materials science researcher at Argonne National Laboratory and at the University of Florida. The team reported the synthesis of borophane in a paper published in Science journal in mid-March.
The scientists claimed that borophane has the same exciting properties as borophene and is stable outside the vacuum chamber. Hersam, who is also director of the Northwestern University Materials Research Science and Engineering Center, explained: “The problem is that if you take borophene out of the ultrahigh vacuum and into air, it immediately oxidises. Once it oxidises, it is no longer borophene and is no longer conductive. The field will continue to be hindered in exploring its real-world use unless borophene can be rendered stable outside an ultrahigh vacuum chamber.”
Although borophene is frequently compared to its super-material predecessor graphene, borophene is much more difficult to create. Graphene is the atomically thin version of graphite, a layered material comprising stacks of two-dimensional sheets. To remove a two-dimensional layer from graphite, scientists simply peel it off.
Boron, on the other hand, is not layered when in bulk form. Five years ago, Hersam and collaborators created borophene for the first time by growing it directly on a substrate. The resulting material, however, was highly reactive, making it vulnerable to oxidation.
Now that borophane can be taken out into the real world, Hersam said researchers will be able to more rapidly explore borophane’s properties and its potential applications.
“Materials synthesis is a bit like baking,” Hersam said. “Once you know the recipe, it’s not hard to replicate. However, if your recipe is just a little off, then the final product can flop terribly. By sharing
the optimal recipe for borophane with the world, we anticipate that its use will rapidly proliferate.”
Have a
story idea?
Tell us.
Do you have a recent research paper or an idea for a science/technology-themed article that you'd like to tell us about?
GET IN TOUCH














